Oxygen Physiology During the Apollo Missions
This week marks the 50th anniversary of one of humanity‘s greatest achievements, the moon landing. A few hundred thousand years after we started processing stones into axes and arrowheads we put three guys in a spaceship and sent the to the next celestial body… amazing, unreal, mind-boggling. I have met people who had traveled to the Congo or to Tibet, distant and interesting places for sure, but there are still a few men walking around today who have traveled to the moon!!! Aaaaamazing!

Apollo 1
An interesting detail of the Apollo missions caught my eye when reading up on the Apollo missions – the breathing gases used during these space flights. As someone interested in scuba diving physiology (where we breathe gases at different pressures as well) it made me think about the breathing mixes used on the way to the moon.
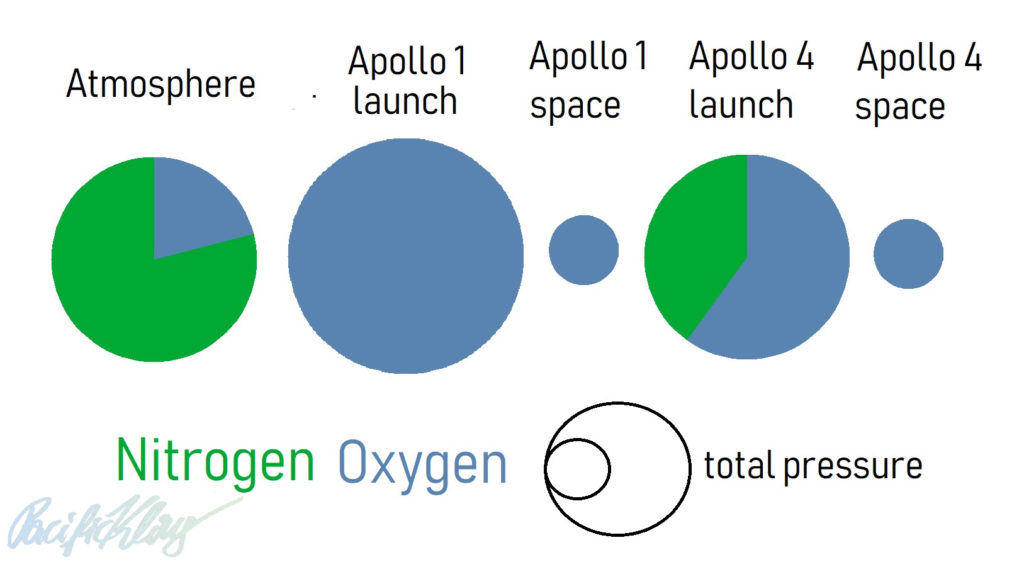
As space-travel aficionados will be aware, sadly a deadly fire killed three astronauts during Apollo 1 while the rocket was still on the ground. The cabin of the space ship during that mission was filled with 100% O2 at a pressure of 1.15 bar. For comparison, the atmosphere at sea level contains 21% oxygen, and most of the rest (~79%) nitrogen, at 1 bar. The oxygen is what our metabolism uses, but it is a very reactive gas (that’s why our metabolism evolved to use it in its biochemistry in first place) and can cause dangerous fires quite easily at high concentrations. Materials which are not dangerous at normal atmospheric partial pressure of oxygen, 0.21, will become flammable in a hyperbaric oxygen atmosphere. An electric spark was all it took under these conditions to cause the tragic accident.
The NASA engineers had decided on using pure oxygen as a breathing gas since the spacecraft had to bring everything the astronauts needed in space – including the gas they breathed. Adding metabolically inert nitrogen (a gas which just goes in and out of the space travelers’ lungs without any metabolic function) would have added extra weight to the spaceship, and made the life support systems more complicated. But excluding nitrogen came at a price – safety. An over-pressure relative to the Earth’s atmosphere was deemed necessary to avoid leak of atmospheric air into the spacecraft before launch.
Once in space, the plan was to keep the atmosphere in the spaceship at 100% oxygen, but to reduce to total pressure to 0.34 bar. This would keep the oxygen partial pressure at 0.34 (100% is equivalent to a fraction of 1.0; 1.0 x 0.34 = 0.34), higher than atmospheric oxygen (0.21), but not overly high, and not a fire hazard.
Engineering Adjustments
The accident at Apollo 1 caused the engineers at NASA to make some modifications to the spaceship. Flammable compounds were eliminated to a large degree, and the breathing gases were changed. From now on, at launch the astronauts would breathe 60% oxygen, 40% nitrogen, at 1 bar.
This initial spaceship-atmosphere would have been significantly enriched in oxygen in contrast to the air humans evolved to breathe. According to the NOAA oxygen tables the single exposure limit for an oxygen partial pressure of 0.6 (= 60% at 1 bar) is 720 minutes (12 hours). anything beyond that can be physiologically harmful.

However, the initial launch phase would have never taken 12 hours, and the astronauts were hence safe oxygen-wise. Again, just as it was planned for Apollo 1, during the later Apollo missions (Apollo 2-3 did not exist as manned missions, the next mission was Apollo 4), cabin pressure was reduced after launch, and the launch cabin atmosphere was replaced with 100% oxygen at 0.34 bar. The difference was that initially the spaceship cabin was filled with more nitrogen and less oxygen, somewhat reducing the fire risk.
What they were breathing in space hence had the a somewhat elevated partial pressure of oxygen compared to the atmosphere (0.34, 34%, versus 21%, 0.21), AND they were breathing it at a reduced total pressure (0.34 bar versus 1 bar). At least in the mid-term (the Apollo 11 mission to the moon took a bit over 8 days), what counts is the partial pressure of oxygen (used by our metabolism), and this value slightly above the physiological normal is probably quite safe for humans, especially for super-fit humans like the Apollo astronauts. Now, the density of a gas can have consequences for its respiratory physiology, notably gases at very high pressures become more viscous and necessitate increased breathing work by the musculature moving our chest and lungs. I am not aware of issues with breathing gases at low pressures over extended periods of time, if there is work on that I would like to learn about it. Would the breathing musculature weakened by this lack of effort necessary after extensive periods of breathing low pressure gases?
This graph shows the breathing gases used in Apollo 1 at launch and in space (projected), and during Apollo 4 and later, at launch and in space:
So!
This small chapter about the technology involved in the Apollo program, and the Apollo 1 accident, shows what an incredibly complex endeavor the trip to the moon was. Every detail had to be thought out, and engineering brains went into every single component of the spaceship. My respect for everyone who was involved in this project is massive!
